Biochemistry of Calcium: Role of Calcium in Muscle Contraction |ChemFam #38|
Greetings to everyone! As we were discussing about biochemistry, this post is no longer exception to that. Today, we are going to discuss about one of the important bio-metals in chemistry i.e., calcium. Calcium is a major component of the structural materials like bone and shell. Formation of skeleton occurs through biomineralization of Ca2+. In such structural units, Ca forms the biominerals like insoluble carbonate (CaCO3, known as calcite) and phosphate [Ca10(PO4)6(OH)2] known as apetite.
calcite is important in shell formation and apetite is involved in the teeth and bone formation. Another biomineral fluoroapetite is required in the formation of enamel on teeth. In fact, in apatite some of the hydroxyl groups are replaced by fluorides to produce fluoroapapatite which is more resistant to the attack of organic acids. Thus the fluoroapatite formation prevents the occurance of dental caries.
Ca2+ also participates in many other biological functions such as muscle contraction, blood clotting, glycolysis, gluconeogenesis and messenger system for hormonal action. Ca2+ is known to have a strong structure forming property. It can activates a number of proteins and enzymes such as secretion of regulating proteins, different phosphatases, protein kinases etc. In some hydrolytic enzyme both Ca2+ and Zn2+ jointly participate. In such causes, Ca2+ probably participates as a structure former. Examples of such hydrolytic enzymes are: neutral proteases and thermolysin which are involved in hydrolysis of peptides, α-amylase involved in hydrolysis of glucosides etc.
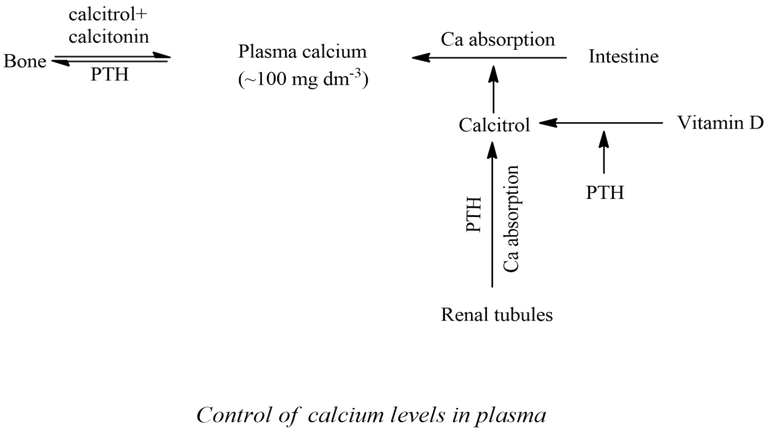
Concentration of Ca2+ in plasma (about 100 mg dm-3) is controlled by the action of three hormones- calcitrol, parathyroid hormone(PTH) and calcitonin(CT). This regulation mechanism is called homeostasis of calcium. Calcitrol promotes the absorption of calcium from the gastrointestinal tract (GI tract) and it also favours the calcification or mineralization of bones. i.e., bone formation. PTH works to elevate the calcium level in plasma in three ways: decalcification or demineralization of bones, Ca reabsorption from kidney tubules, absorption of the Ca from the GI tract by favouring the synthesis of calcitrol from vitamin D. Calcitonin favours the calcification process and thus it reduces The Ca level in plasma. The action of PTH is antagonistic to that of CT.
Deficiency of Calcium leads to Diseases
Rickets(defective calcification) arises due to low level of vitamin D or low intake of Ca or P or both. It mainly occurs among the children. Osteoporosis(demineralization of bones) involves weakening of bone. Impaired functioning of vitamin D and other hormones are responsible for this disease. This disease occurs mainly among the aged people.Generally with aging the bones start to decay.
In hypercalcemia, the serum calcium level is increased due to the hyperactivity of the parathyroid glands. The involved clinical symptoms are : increase of alkaline phosphatase activity, increase in urinary excretion of calcium and phosphorous leading to the formation of kidney stones. Hypocalcemia leads to fall in serum calcium level. It produces tetany whose clinical symptoms are : spasms and convulsions and neuromuscular irritation. In fact, hypocalcemia is more dangerous than hypercalcemia.
Binding sites of Calcium in Proteins
Ca2+ is hard centre and it prefers the hard donor sites like O. The Ca2+ binding sites generally involve the carboxylate groups of aspartate and glutamate residues. The ligand γ-carboxyglutamate having two carboxylate groups (hard anionic binding sites) for coordinating Ca2+. Ca2+ very often exists as CaO6 or CaO7 or CaO7. Thus like Na+ and K+ ions, Ca2+ ion also prefers higher coordination number. The preference of coordination number for Ca2+ follows the sequence : 8>7>6>9.
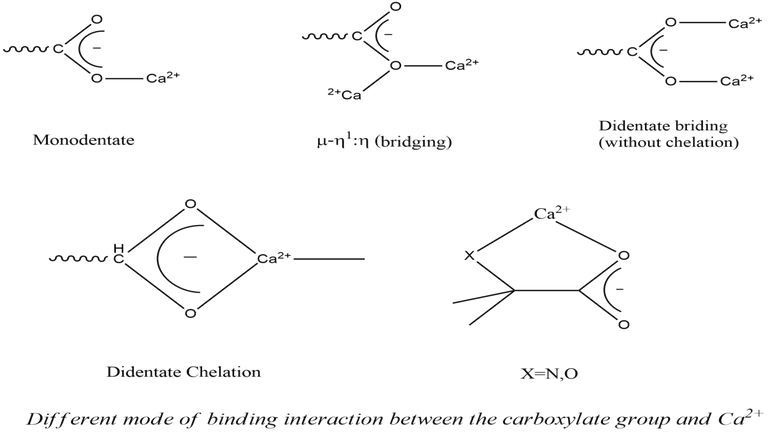
Among the different possible Ca2+ binding sites, the carboxylate groups of side chains of amino acids are the most preferred sites. Carboxylate groups can bind with the Ca2+ ion in different possible ways. Nature has developed two novel amino acids (gla and hya) which are selectively used as strong chelators of Ca2+ in blood clotting process. Incorporation of a -COOH group at the &gamma: carbon of glutamic acid gives gla (γ-carboxyglutamic acid) and incorporation of a -OH group at the β-C of aspartic acid gives hya (β-hydroxyaspartic acid).
Calcium in Muscle Contraction
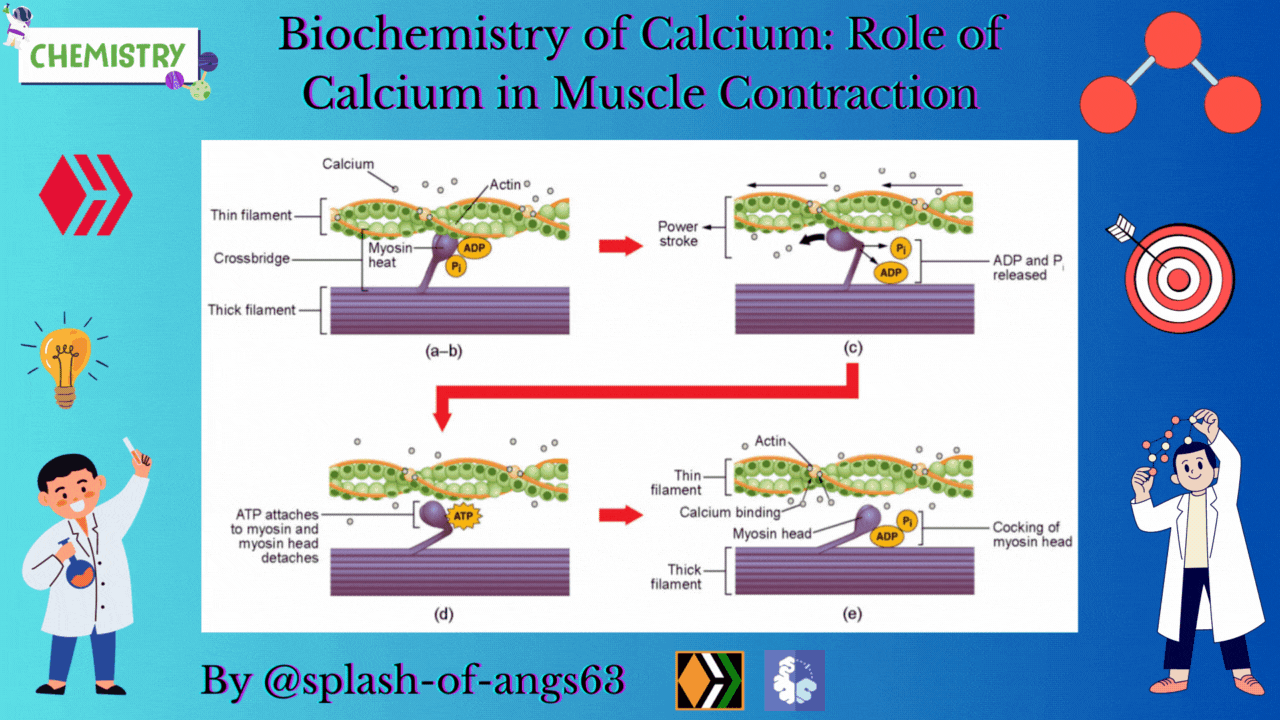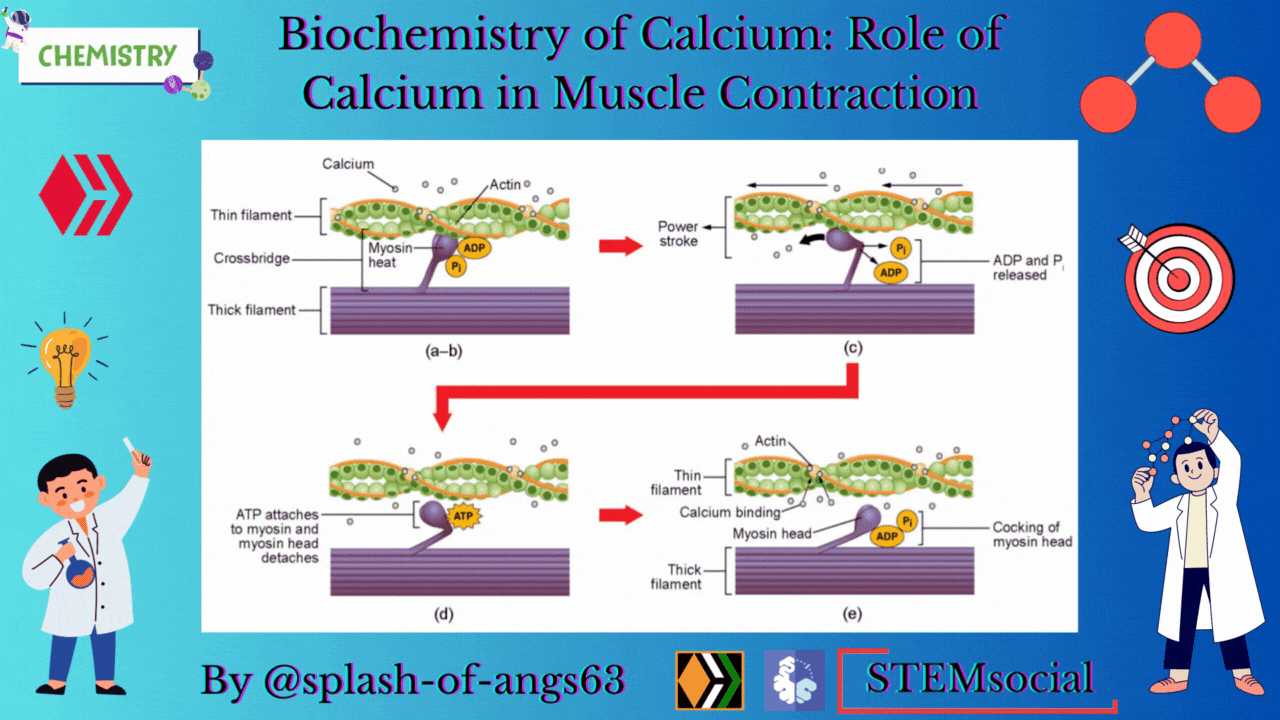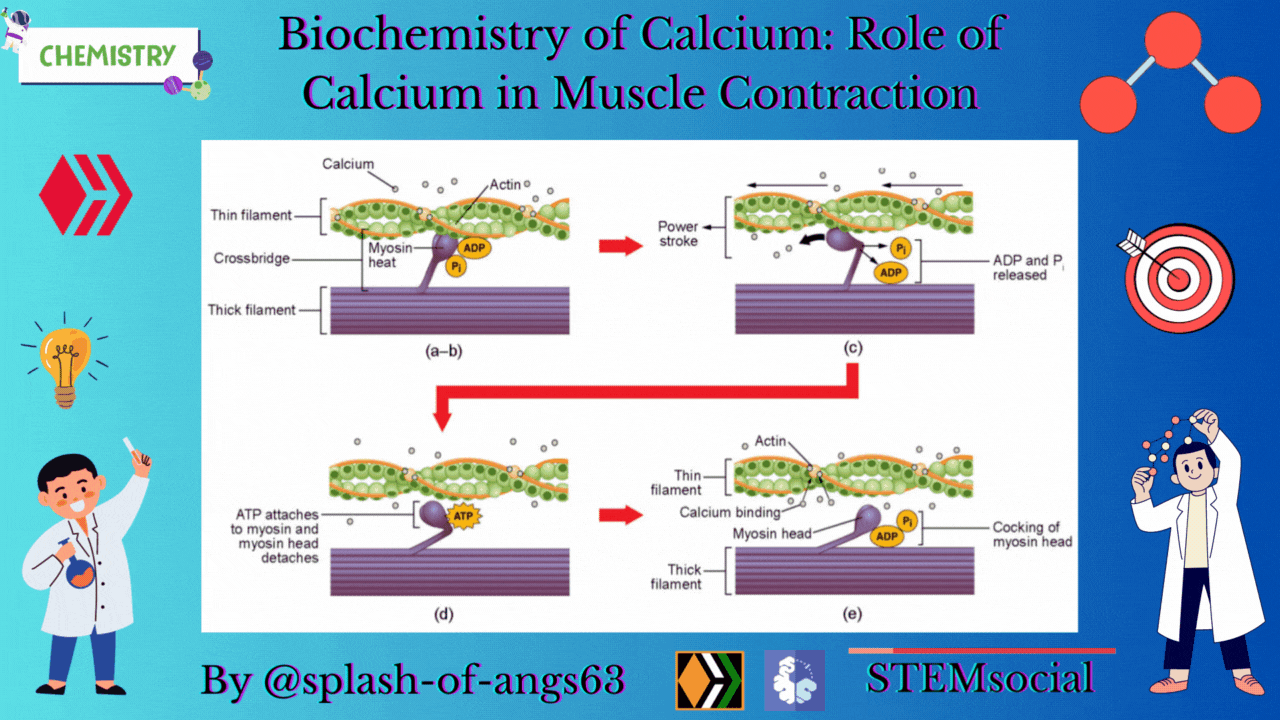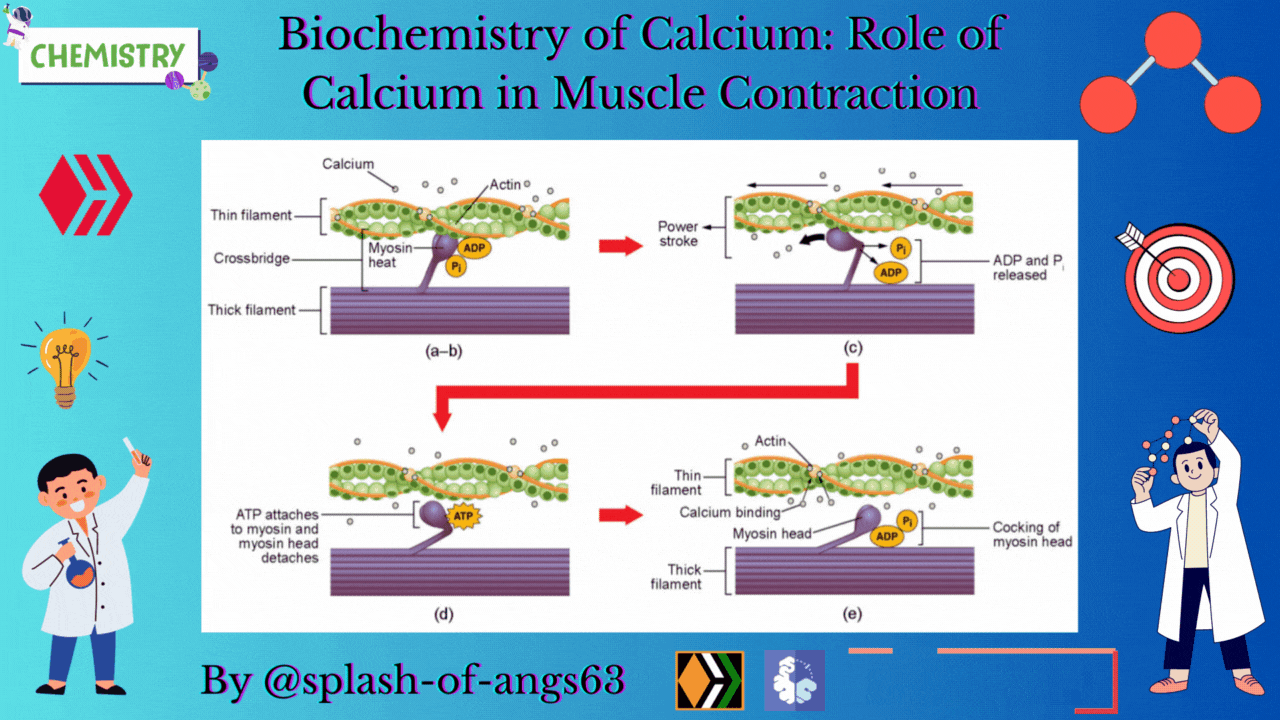
The endoplasmic reticulum i.e., sarcoplasmic reticulum (SR) of the muscle fibres is the store house of Ca2+ ions. Troponin and tropomyosin are Ca2+ binding proteins in muscle fibres. The SR, troponin and tropomyosin jointly act to execute the muscle contraction.
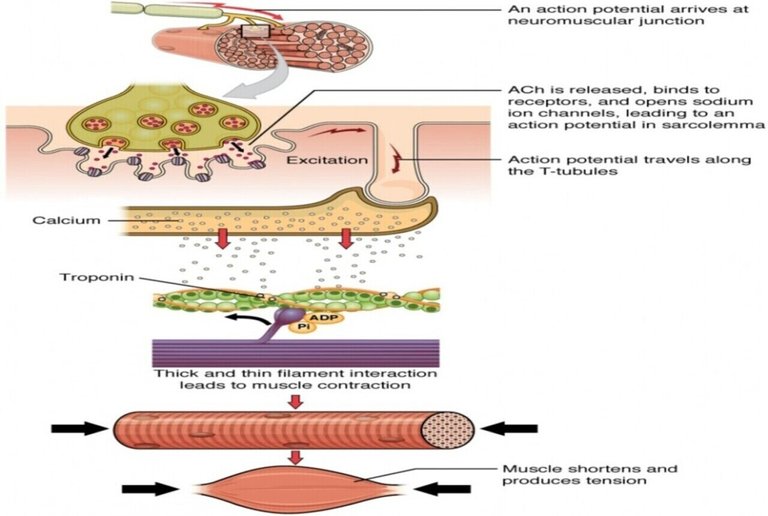
The striated appearance of muscle fibres is due to the distribution pattern of two important proteins - actin and myosin. The interaction between action and myosin is mediated by tropomyosin and troponin complex.
Tropomyosin is a double helix protein. Troponin consists of three polyketide chains- troponin-C (Tn-C), troponin-I (Tn-I) and troponin-T (Tn-T). Tn-C is the calcium binding site. Tn-I binds with actin and Tn-T binds with tropomyosin.
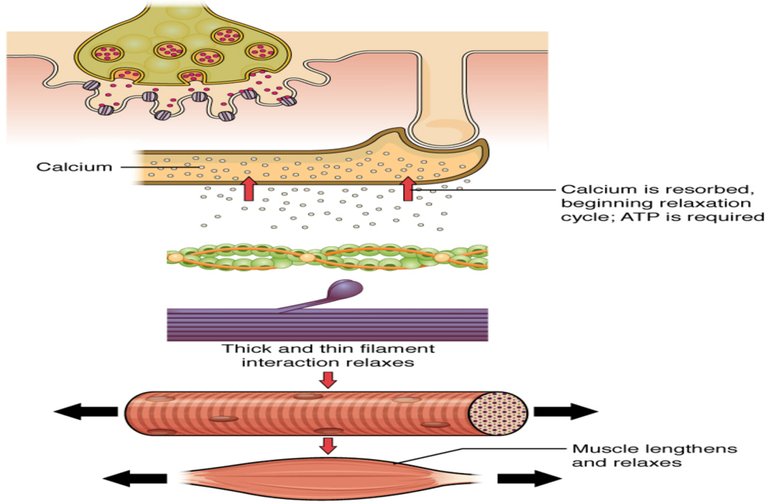
Nerve excitation triggers the release of Ca2+ from SR to the surrounding muscle fibres. These released Ca2+ ions bind with troponin at Tn-C site and this binding cause a conformational change. Structural change in Tn-C also changes the structure of Tn-I. This change pulls the Tn-I away from the actin and consequently it uncovers the myosin binding sites on actin. The structural change in Tn-C is also transmitted to tropomyosin through Tn-T. Due to the conformational change in tropomyosin, the blocking of the active site in actin by tropomyosin is removed. Then myosin can interact with the active sites of actin of the filament. It generates the contractile force. Again the Ca2+- pump transports the Ca2+ ions into SR and consequently the interaction between actin and myosin is stopped at the resting condition.

So, we have discussed about the crucial roles of calcium in biological system and thoroughly gone through the role of calcium in muscle contraction. We shall meet again. Happy learning!
Muscle fibre contraction and relaxation
Calcium regulation of muscle contraction
Biosynthesis of Fatty Acids: De Novo Synthesis of Fatty Acids |ChemFam #37|
Hapticity and The Eighteen Electron Rule |ChemFam #36|
An Introduction To Organometallic Chemistry |ChemFam #35|
Applications of Zeolites: The 3D Molecular Sieves |ChemFam #34|
Properties of Zeolites: The 3D Molecular Sieves |ChemFam #33|
Zeolites: The 3D Molecular Sieves |ChemFam #32|
The Beauty of Eucalyptus Tree |ChemFam #31|
The Accidental Cure for Cancer: Cisplatin |ChemFam #29|
Acceptorless Dehydrogenation and Related Transformations |ChemFam #28|
PS The thumbnail image is being created by me using canva.com by taking template image from Anatomy and physiology



This post has been manually curated by @bhattg from Indiaunited community. Join us on our Discord Server.
Do you know that you can earn a passive income by delegating to @indiaunited. We share more than 100 % of the curation rewards with the delegators in the form of IUC tokens. HP delegators and IUC token holders also get upto 20% additional vote weight.
Here are some handy links for delegations: 100HP, 250HP, 500HP, 1000HP.
100% of the rewards from this comment goes to the curator for their manual curation efforts. Please encourage the curator @bhattg by upvoting this comment and support the community by voting the posts made by @indiaunited.
Congratulations @splash-of-angs63! You have completed the following achievement on the Hive blockchain And have been rewarded with New badge(s)
Your next target is to reach 6000 upvotes.
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPTo support your work, I also upvoted your post!
Check out our last posts:
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support.