Understanding Nucleophilic Substitution Reactions: Exploring SN1 and SN2 | ChemFam #90|
*Greetings everyone! We have studied about haloalkanes previously. One of the reaction these haloalkanes shows is a substitution type reaction. Nucleophilic substitution reactions are fundamental transformations in organic chemistry where one nucleophile replaces another atom or group in a molecule. These reactions are essential in synthesizing various organic compounds, pharmaceuticals and understanding biological processes. *
The halogen atom in haloalkanes (alkyl halide) is more electronegative than the carbon atom attached to it. As a result, carbon atom acquires a partial positive charge and the halogen atom a partial negative charge.
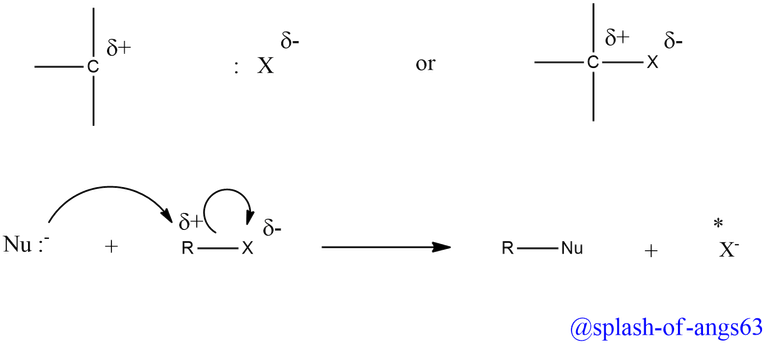
The presence of this small positive charge on the carbon atom makes it susceptible to attack by nucleophilic reagents (i.e. reagents with a lone pair of electron or a negative charge). As a result, when a nucleophile stronger than the halide ion approaches the positively charged carbon atom of an alkyl halide, the halogen atom and its electron bonding pair are displaced and a new bond is created between the carbon atom and the incoming nucleophile.
Such reactions in which a stronger nucleophile displaces a weaker nucleophile are called nucleophilic substitution reactions and the atom or group (halide ion in the given example) which departs with its bonding pair of electrons is called the leaving group. Better the leaving group, more facile is the nucleophilic substitution reaction.
Mechanism of nucleophilic substitution reactions
There are two types of nucleophilic substitution reactions. these are:
(a) SN2 (substitution, nucleophilic, bimolecular)
(b) SN1 ( substitution, nucleophilic, unimolecular)
- (a) Substitution nucleophilic bimolecular SN2
The reaction between between methyl chloride and hydroxide ion to yield methanol and chloride ion follows second order kinetics, i.e., the rat of the reaction depends upon the concentration of both the product and reactants. This simply implies that both the alkyl halide and the nucleophile OH- are taking part simultaneously in the rate determining step of the reaction.
In other words, when the incoming nucleophile, OH- ion approaches the alkyl halide and starts interacting with it, the carbon halogen bond starts breaking and a new carbon-OH bond starts forming. These two processes takes simultaneously in a single step and no intermediate is formed. Thus, SN2 reactions are concerted in nature, i.e., take place in one step. Such reactions occur through a transition state in which both the reactants are partially bonded to each other as shown below:
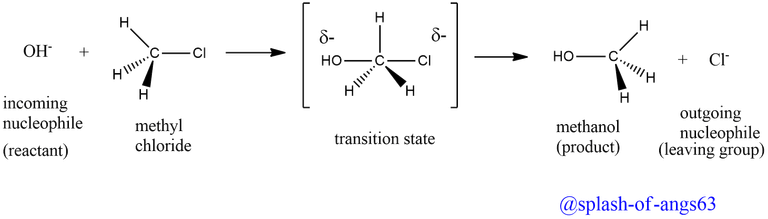
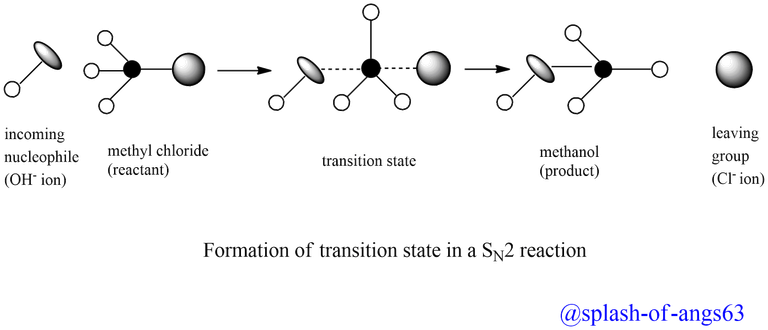
In the transition state, the carbon atom forms simultaneous bonds with the departing group, Cl-, and the arriving nucleophile, OH- ion. In other words, in the transition state, the carbon atom is bonded to five atoms and thus the transition state is unstable and hence can not be isolated. Being unstable, it ultimately decomposes to form the product (CH3OH) and the leaving group, Cl- ion.
It is interesting to note that in SN2 reactions, the attack of the nucleophile occurs from the back side and the leaving group leaves from the back side. As a result of this, SN2 reactions are invariably accompanied by inversion of configuration, much to how an umbrella in a high wind turns inside out. Walden inversion is the term for this inversion of configuration.
- (b) Substitution nucleophilic bimolecular SN1
SN1 reactions are generally carried out in polar protic (hydroxylic nature) solvents. Example includes water, alcohol, acetic acid etc. The reaction between tert-butyl bromide and the OH- ion to yield tert-butyl alcohol and Br- ion follows first order kinetics, i.e., the rate of the reaction depends upon the concentration of tert-butyl bromide only and is independent of the concentration of OH- ion.
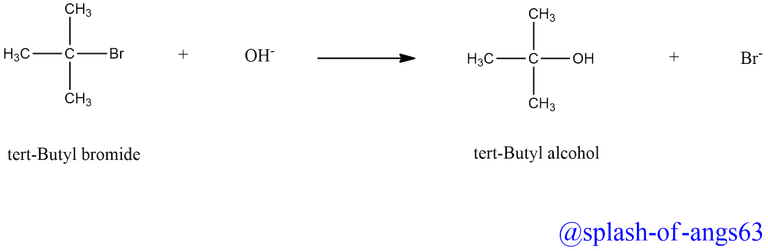
This suggests that the reaction completion involves two steps . In the first step, tert-butyl bromide undergoes ionization to produce tert-butyl carbocation and a bromide ion.
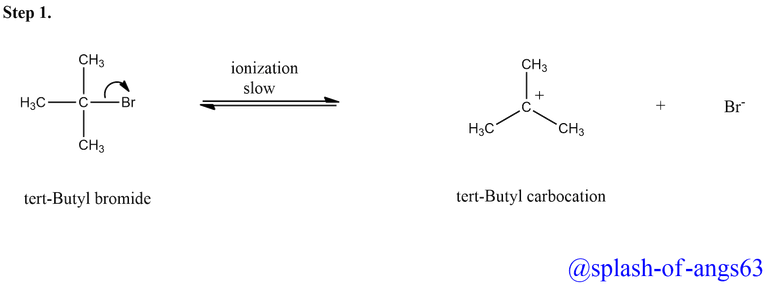
The energy needed for the cleavage of the C-Br bond is obtained through the solvation of the bromide ion with the proton of the protic solvents. This step is slow and reversible and hence is the rate-determining step of the reaction.
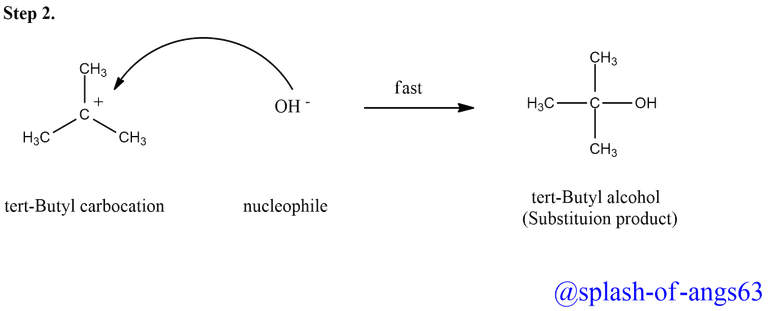
In the second step, the carbocation being a reactive chemical species, is immediately attacked by the nucleophile, i.e., OH- ion to give substitution product (tert-butyl alcohol). This step is fast and hence does not not affect the rate of the reaction.

Software used:
The chemical reactions and diagrams are drawn using ChemDraw software.
PS All the images used in this blog are my original work and belongs to me unless and otherwise separately mentioned
If you like my work and would like to support me, you can do so by joining my fanbase by clicking this link
Understanding Basic Principle and Theory of Mass Spectrometry | ChemFam #89 |
Blood Substitutes: A Quest for Artificial Blood | ChemFam #88 |
Properties of Haloalkanes and Methods of Preparation of Haloalkanes | ChemFam #87 |
Understanding the Simple Harmonic Oscillator in Quantum Mechanics | ChemFam #86 |
Understanding Degeneracy in Quantum Chemistry: Exploring 1D, 2D and 3D Box Models | ChemFam #85 |
Introduction to Zero Point Energy and its Cases in Particle in a Box | ChemFam #84 |
Quantum Confinement : Particle in a 2D box and 3D box | ChemFam #83 |
Molecular Chirality: A Mirror Image Perspective | ChemFam #82|
Exploring Time-Independent Schrödinger's Wave Equation and Particle in a 1D Box | ChemFam #80 |
The Role of Gamma Function in Quantum Mechanics | ChemFam #79 |
Postulates of Quantum Mechanics and Normalization of Wavefunction |ChemFam #78|
Understanding Commutator Relations and Exploring Eigenfunctions in Quantum Mechanics |ChemFam #77|
How to find Expression of an Operator and Commutation Relations |ChemFam #76|
Basics to Quantum Chemistry: Operators, Functions and Properties of Operators |ChemFam #75|
A Comprehensive Study of Euler's Reciprocal Rule in Thermodynamics |ChemFam #73|
PS The thumbnail image is being created by me using canva.com
Games I play on Hive
| Games I play on Hive | Game description |
|---|---|
| Terracore | Terracore is an Idle mining game based on Hive blockchain |
| Rise of the Pixels | ROTP is a Web3 browser game about game development on Hive |

Posted Using InLeo Alpha



This post has been manually curated by @steemflow from Indiaunited community. Join us on our Discord Server.
Do you know that you can earn a passive income by delegating to @indiaunited. We share more than 100 % of the curation rewards with the delegators in the form of IUC tokens. HP delegators and IUC token holders also get upto 20% additional vote weight.
Here are some handy links for delegations: 100HP, 250HP, 500HP, 1000HP.
100% of the rewards from this comment goes to the curator for their manual curation efforts. Please encourage the curator @steemflow by upvoting this comment and support the community by voting the posts made by @indiaunited..
This post received an extra 1.06% vote for delegating HP / holding IUC tokens.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support.
!hiqvote
@splash-of-angs63, the HiQ Smart Bot has recognized your request (1/3) and will start the voting trail.
In addition, @splash-of-angs63 gets !LOL from @hiq.redaktion.
For further questions, check out https://hiq-hive.com or join our Discord. And don't forget to vote HiQs fucking Witness! 😻
lolztoken.com
This changes everything.
Credit: marshmellowman
@splash-of-angs63, I sent you an $LOLZ on behalf of hiq.smartbot
(4/8)
Delegate Hive Tokens to Farm $LOLZ and earn 110% Rewards. Learn more.