Understanding the Significance of Wavefunction and Wavefunction Plots for Particle in a 1D Box | ChemFam #81|
Greetings to everyone! In my previous blog, we have studied about Schrodinger's wave equation and derived a solution to it by considering a one dimensional system. We were able able to find the value of normalization constant and also successfully found the energy eigenvalue. We almost completed the 1D box, just I thought, I need to give a visual representation of wavefunction plots for 1D box. This way, we will be able to grasp the concepts better. So, today, we shall be discussing about wavefunction plots for 1D box.
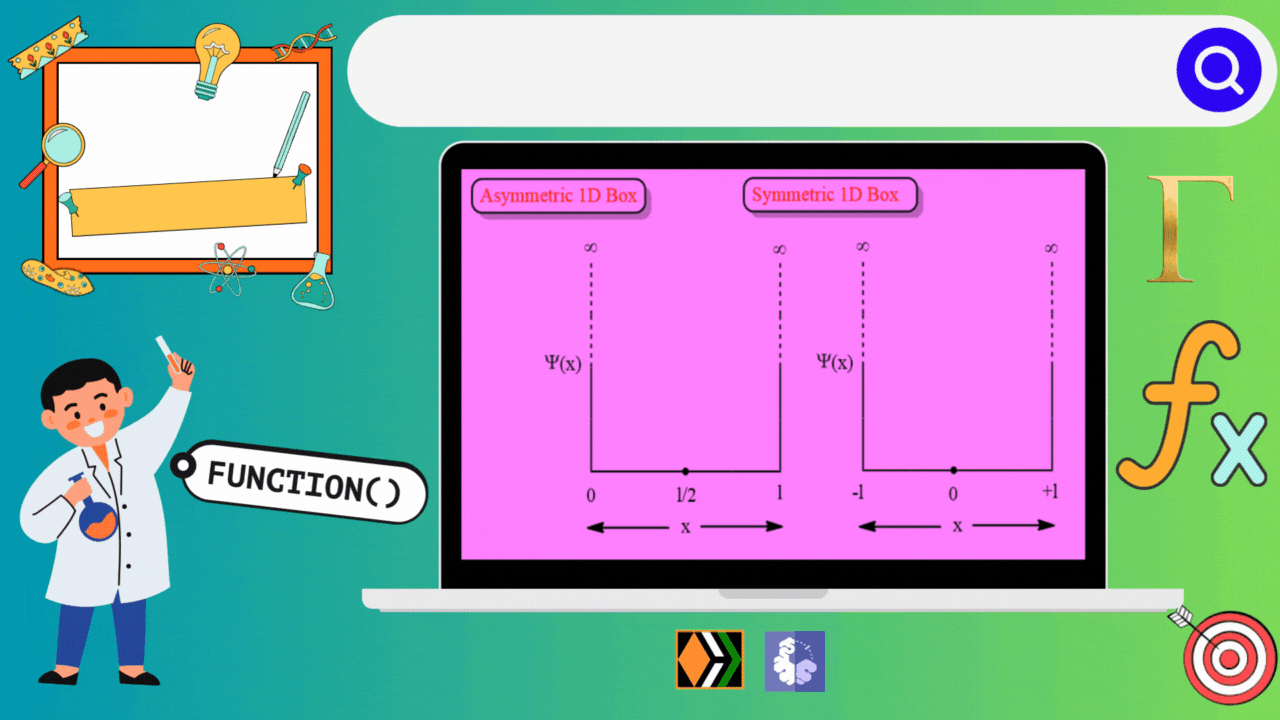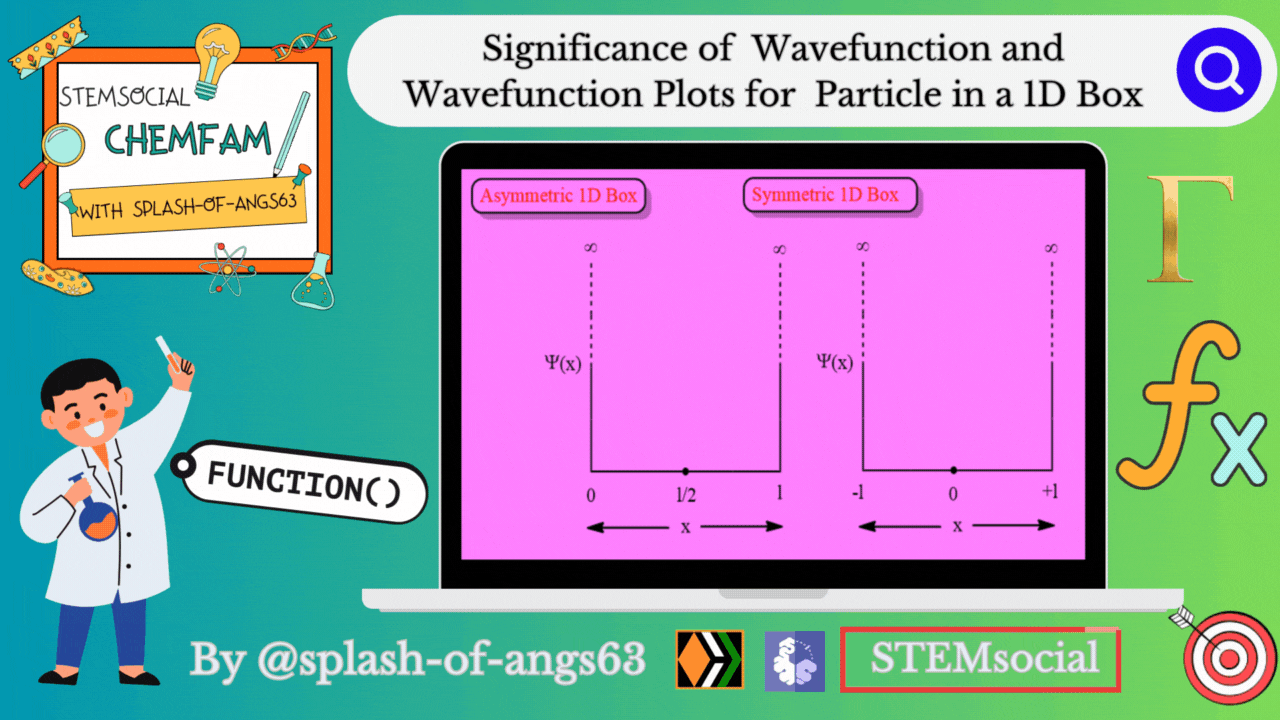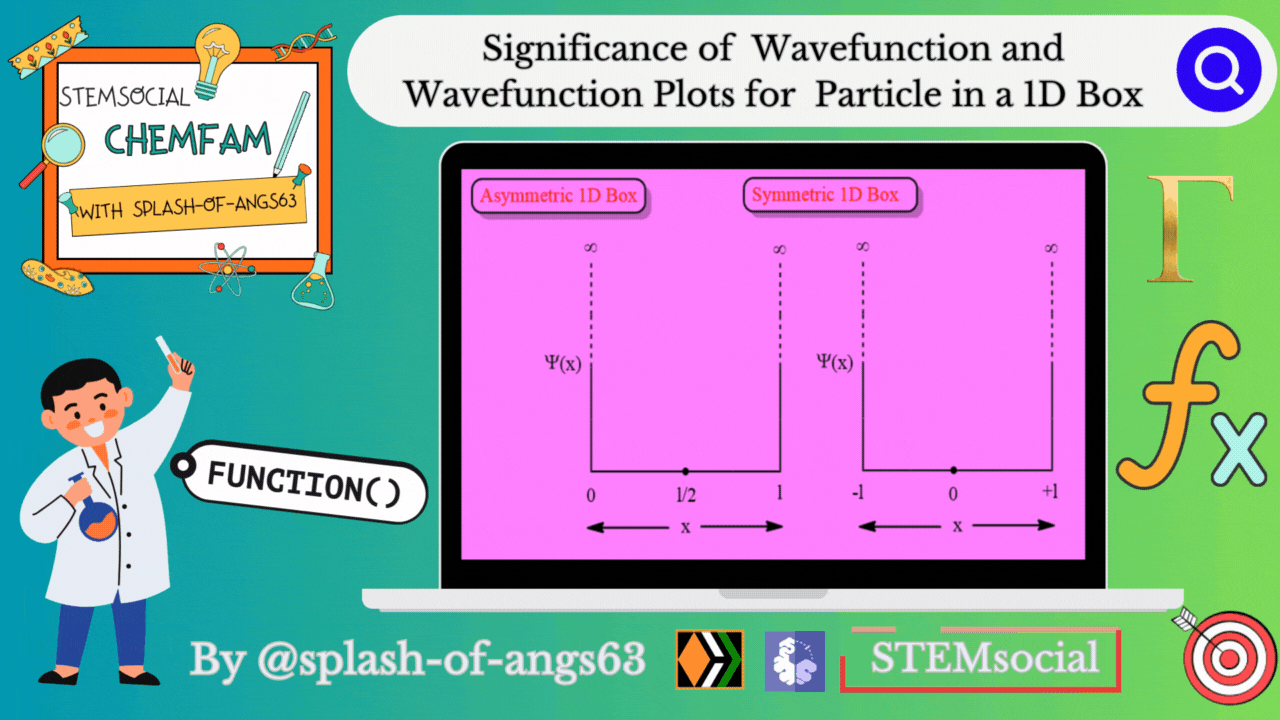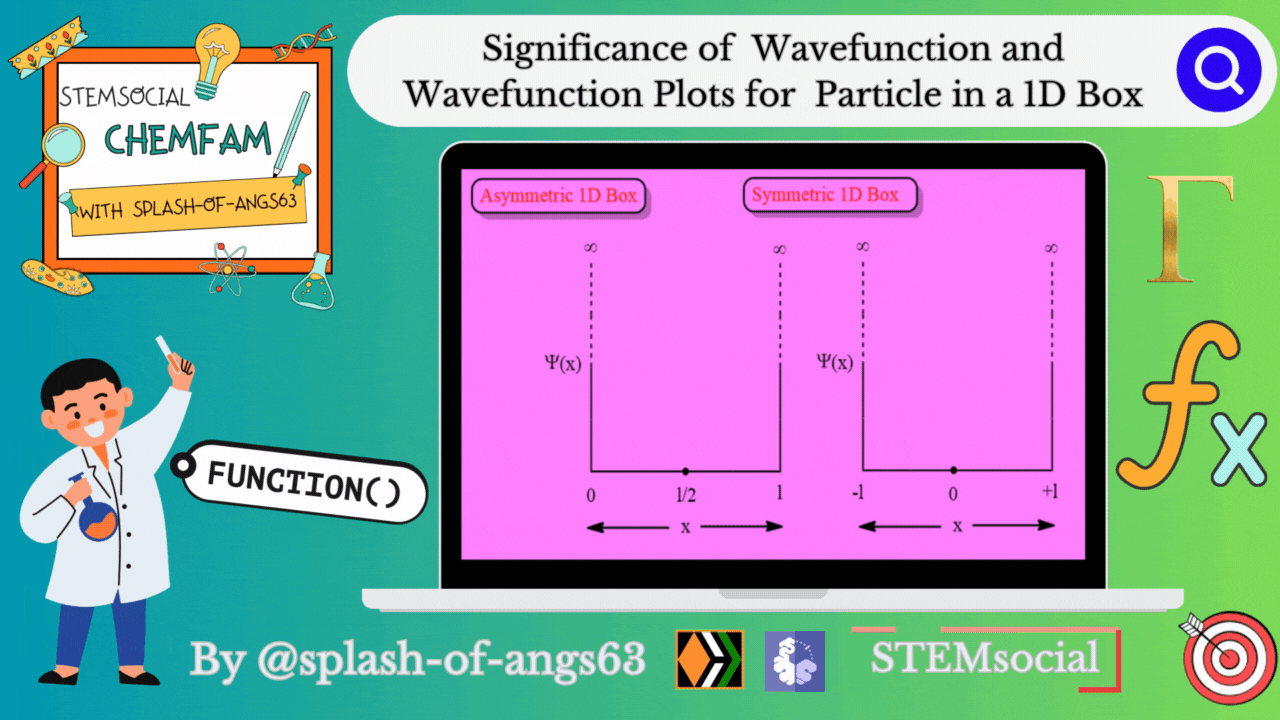
Significance of Wavefunction
In quantum mechanics, the wavefunction is a mathematical function that represents the quantum state of a particle. The square of the wavefunction, denoted as |ψ|², gives the probability density of finding the particle at a particular position. The wavefunction contains information about the particle's position, momentum, and other observable properties.
The wavefunction, often represented by the symbol ψ, evolves over time according to the Schrödinger equation, a fundamental equation in quantum mechanics. By solving the Schrödinger equation for specific systems, scientists can obtain wavefunctions that describe the behavior of particles in those systems.
- Ψ actually has no physical significance.
- Ψ2 gives the probability of finding electron in a certain area.
- ΨΨ* dτ gives the probability of finding the electron in a small volume dτ
- ΨΨ* is always positive and real.
Important note
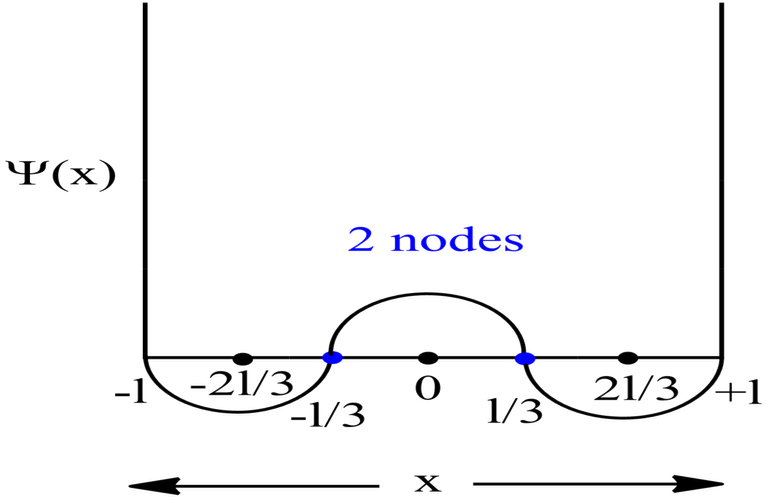
When Ψ2 value is very high, it means that the probability of finding the electron is very high in those regions. These regions are therefore termed as atomic orbitals. Now, the regions corresponding to Ψ2 value equal to 0, are the space where probability of finding the electron is zero i.e., null. These regions are called as node(singular) or nodes(plural).
Wavefunction Plots for the 1D Box:
To gain insights into the behavior of a particle in a 1D box, physicists often rely on graphical representations of the wavefunction. These plots provide a visual understanding of how the probability density changes along the confined space.
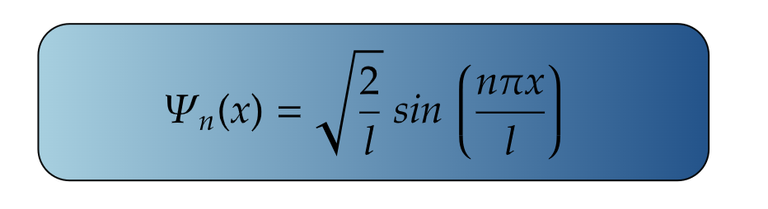
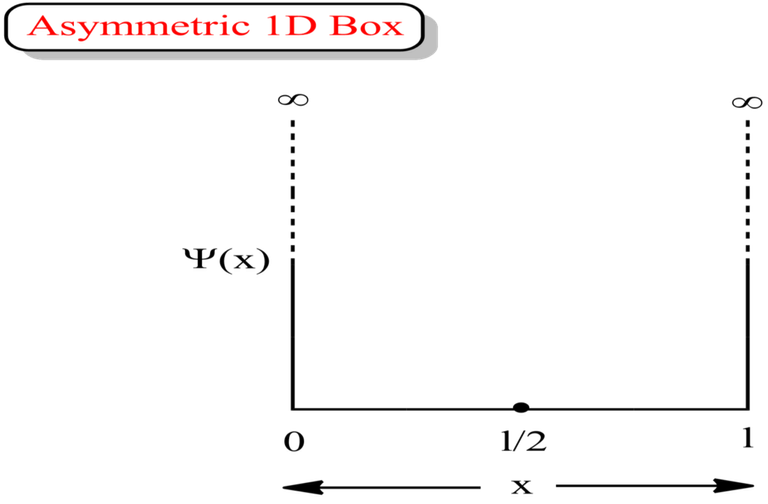
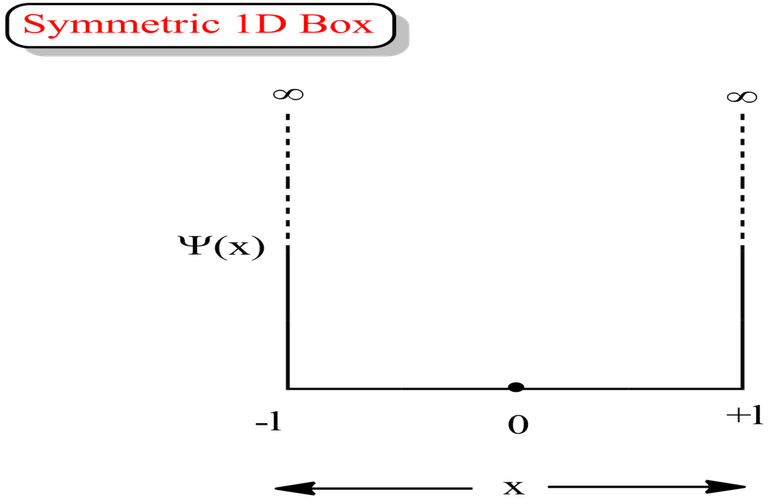
Asymmetric 1D Box
For Ground State
It is important to note that in particle in a box, ground state belongs to the state where n=1 and not n=0.
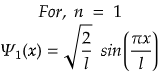
For maximum value, sin(πx/l) should be maximum.
i.e., at sin 90°=1= sin(π/2)
So,
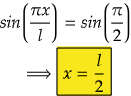
Therefore, at x=l/2, the value of wavefunction ψ1(x) will be maximum.
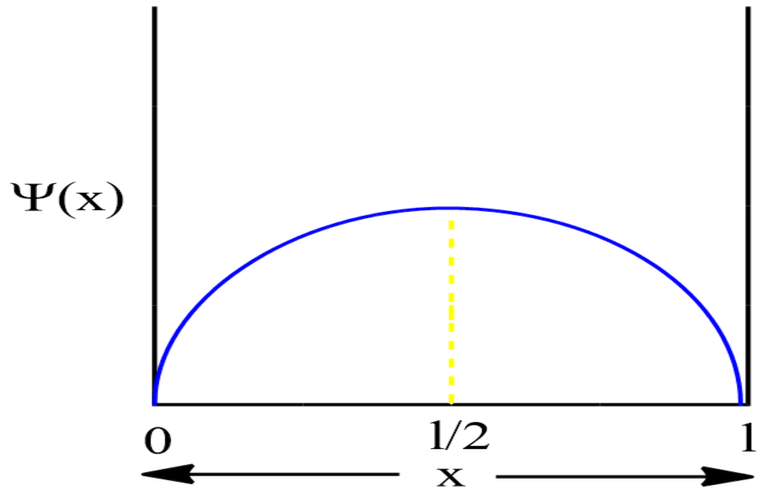
For First Excited State
n=2 for first excited state
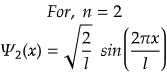
So, for maximum value again, sin(2πx/l) should be maximum.
i.e., at sin 90°=1= sin(π/2)
So,
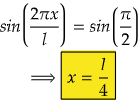
Therefore, at x=l/4, the value of wavefunction ψ2(x) will be maximum.
Now, for x=l/2,

Again for x=3l/4,
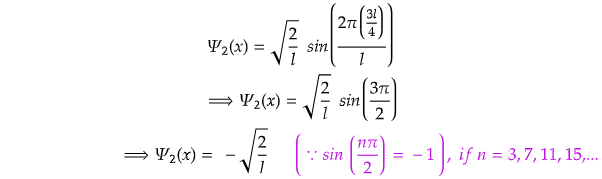
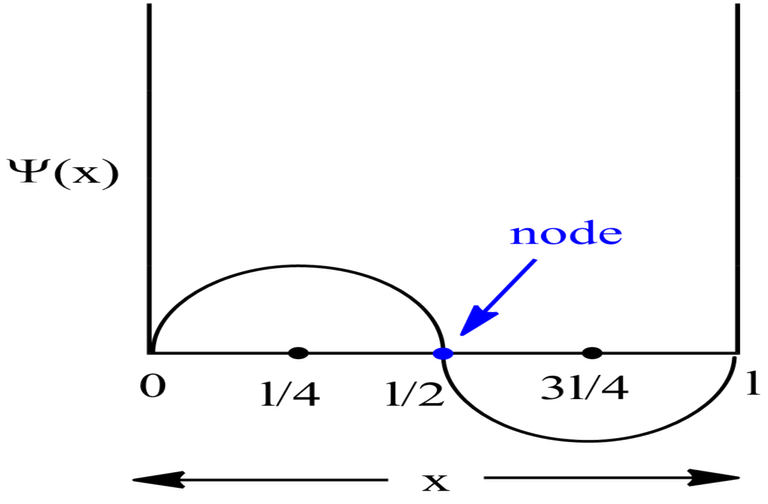
For Second Excited State
n=3 for second excited state.
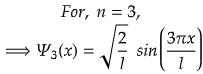
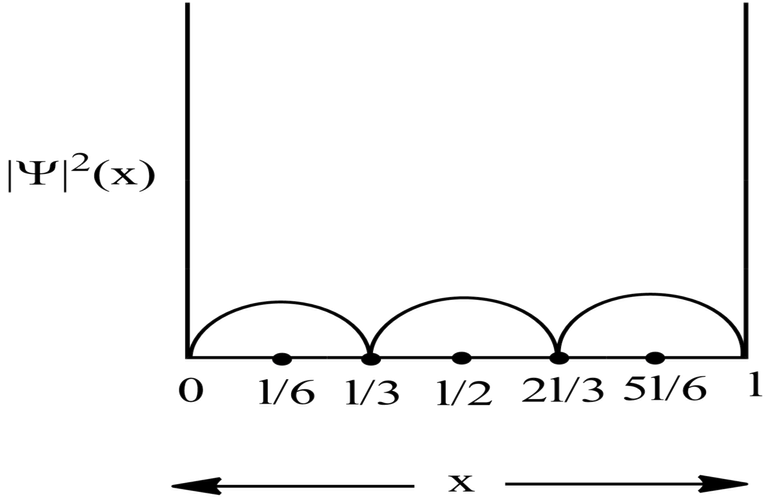
So, for maximum value again, sin(3πx/l) should be maximum.
i.e., at sin 90°=1= sin(π/2)
So,
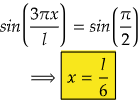
Therefore, at x=l/6, the value of wavefunction ψ3(x) will be maximum.
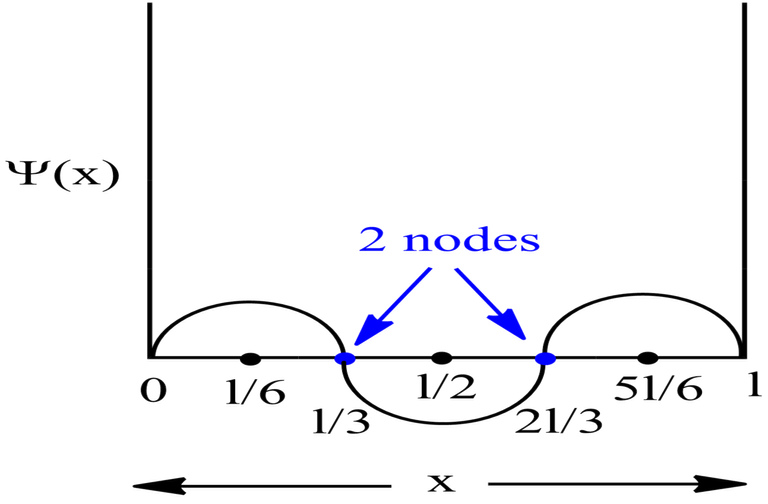
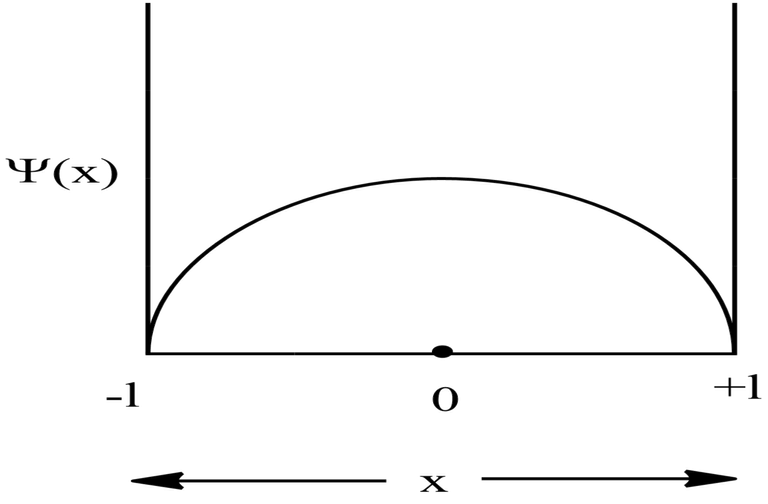
Probability Plots
|Ψ|2 is definitely a positive quantity, so the plots will lie on or above the positive axes only.
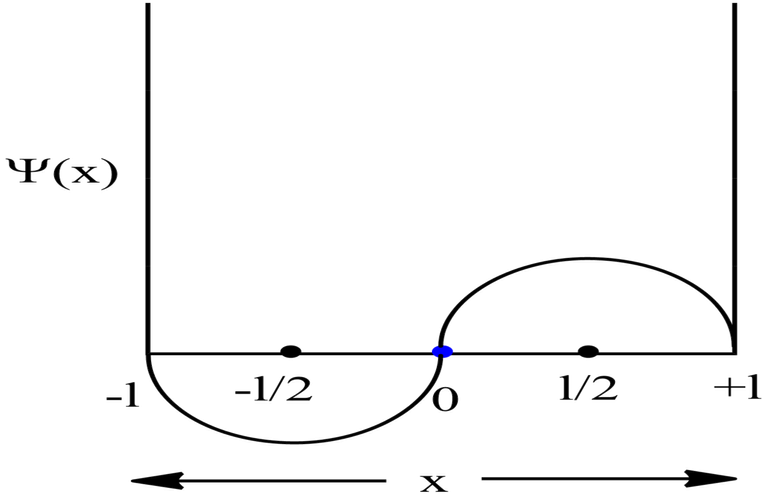
Symmetric 1D Box
Always go with the formulas for asymmetric box, if the question does not mention whether the box is asymmetric or symmetric 1D box.
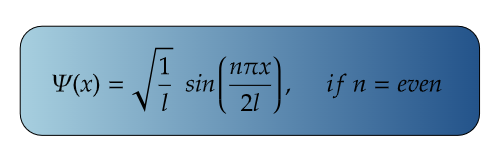
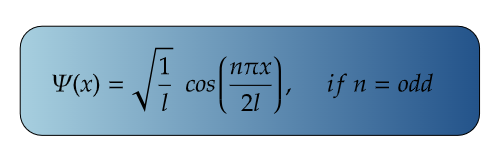

For Ground State
For, ground state, n=1,
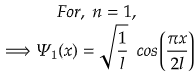
For maximum value, cos(πx/2l) should be maximum.
i.e., at cos 0°=1= maximum
So,
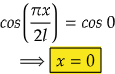
Therefore, at x=0, the value of wavefunction ψ1(x) will be maximum.
For First Excited State
n=2 for first excited state
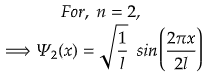
So, for maximum value again, sin(2πx/2l) should be maximum.
i.e., at sin 90°=1= sin(π/2)
So,
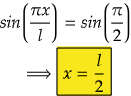
Therefore, at x=l/2, the value of wavefunction ψ2(x) will be maximum
For Second Excited State
n =3 for second excited state
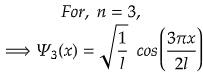
So, for maximum value again, cos(3πx/2l) should be maximum.
i.e., at cos 0°=1= maximum
So,
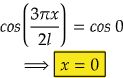
Therefore, at x=0, the value of wavefunction ψ3(x) will be maximum.
What we learnt?
We studied the significance of wavefunction, the probability of finding an electron in a certain region. We were able to understand what are nodes and what are atomic orbitals.
We studied the wavefunction plots for particle in a asymmetric one dimensional box. We studied the ground state, first excited state and the second excited state and found out the length of the box for which wavefunction will be maximum.
We studied the wavefunction plots for particle in a symmetric one dimensional box. We studied the ground state, first excited state and the second excited state and found out the length of the box for which wavefunction will be maximum.
Software used:
The mathematical equations are prepared using mathcha.io editor and diagrams are drawn using ChemDraw software.

If you like my work and would like to support me, you can do so by joining my fanbase by clicking this link
Exploring Time-Independent Schrödinger's Wave Equation and Particle in a 1D Box | ChemFam #80 |
The Role of Gamma Function in Quantum Mechanics | ChemFam #79 |
Postulates of Quantum Mechanics and Normalization of Wavefunction |ChemFam #78|
Understanding Commutator Relations and Exploring Eigenfunctions in Quantum Mechanics |ChemFam #77|
How to find Expression of an Operator and Commutation Relations |ChemFam #76|
Basics to Quantum Chemistry: Operators, Functions and Properties of Operators |ChemFam #75|
A Comprehensive Study of Euler's Reciprocal Rule in Thermodynamics |ChemFam #73|
A Deep Dive into Nutrition Essentials: Your Path to a Healthier, Happier You |ChemFam #72|
Decoding Liver Function Tests through Chemistry |ChemFam #71|
Understanding the Dynamic Roles of Metalloenzymes and Metal-Activated Enzymes |ChemFam #70|
Cracking the Thermal Code: Differential Thermal Analysis in Modern Research |ChemFam #69|
Applications and Importance of IR Spectroscopy: Shedding Light on Molecular Structures |ChemFam #68|
The Silent Revolution: How Polymers are Shaping Our World? |ChemFam #67|
Beyond the Bin: The Many Faces of Plastic Management |ChemFam #66|
Spectrophotometry Simplified: The Beer-Lambert Law in Spectrophotometry |ChemFam #65|
Chromatography: Unraveling the Science of Separation |ChemFam #64|
Colorful Clues: The Magical World of Chemical Indicators |ChemFam #63|
Colloids in Action: Impacting Your Daily Life More Than You Think |ChemFam #62|
The Complex Landscape of Opioid Analgesics: Addressing The Concerns |ChemFam #61|
Genetic Engineering: Pioneering Progress or Ethical Predicament? |ChemFam #60|
The Guardians Against Microbial Menace: Antibacterial Agents |ChemFam #59|
The Cholesterol Conundrum: The Story of Statins |ChemFam #58|
PS The thumbnail image is being created by me using canva.com
Games I play on Hive
| Games I play on Hive | Game description |
|---|---|
| Terracore | Terracore is an Idle mining game based on Hive blockchain |
| Rise of the Pixels | ROTP is a Web3 browser game about game development on Hive |



Congratulations @splash-of-angs63! You have completed the following achievement on the Hive blockchain And have been rewarded with New badge(s)
Your next target is to reach 800 comments.
Your next target is to reach 45000 upvotes.
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPTo support your work, I also upvoted your post!
Check out our last posts:
!hiqvote
!HBIT
@splash-of-angs63 mined HBIT. ⛏️ (1/1) tools | trade | connect
Made with LUV by crrdlx.
@splash-of-angs63, the HiQ Smart Bot has recognized your request (1/2) and will start the voting trail.
In addition, @splash-of-angs63 gets !WEED from @hiq.redaktion.
For further questions, check out https://hiq-hive.com or join our Discord. And don't forget to vote HiQs fucking Witness! 😻
@splash-of-angs63!
@hiq.smartbot passed you the virtual joint!If you do not want to receive these comments, please reply with !STOP
This post has been manually curated by @bhattg from Indiaunited community. Join us on our Discord Server.
Do you know that you can earn a passive income by delegating to @indiaunited. We share more than 100 % of the curation rewards with the delegators in the form of IUC tokens. HP delegators and IUC token holders also get upto 20% additional vote weight.
Here are some handy links for delegations: 100HP, 250HP, 500HP, 1000HP.
100% of the rewards from this comment goes to the curator for their manual curation efforts. Please encourage the curator @bhattg by upvoting this comment and support the community by voting the posts made by @indiaunited..
This post received an extra 1.05% vote for delegating HP / holding IUC tokens.